A newly announced X-Prize aims to address the increasing acidity in the world’s oceans.
A Two Split Prize
The prize is a two split 2 million dollar competition, with the first million to be awarded in 2015 to the team that has developed an accurate deep-water acidity monitor, and another million for having developed an inexpensive shallow-water monitor.
Carbon Dioxide to Carbonic Acid
Since the oceans are an important part of the planet’s carbon cycle and absorbs high amounts of the carbon from the atmosphere (an estimated 23 percent of man-made CO2 emissions) – the burning of fossil fuels and increase of carbon dioxide in the atmosphere thus also increase the amount of carbon absorbed by the oceans – and this fact is slowly shifting the pH level in the oceans.
Higher carbon dioxide levels in the air increase the acidity (decrease in the pH) of the Earth oceans via simple chemical reactions. When carbon dioxide dissolves in water (with sunlight), it reacts to form free carbon oxide CO, carbonic acid (H2CO3), bicarbonate (HCO−3) and carbonate (CO2−3).
The fact that some of the carbon dioxides end up as excess carbonic acid absorbed into seawater is something we have known for long, but researchers don’t have exact data on how, where and how much. This is much because of a shortfall in cheap, accurate and reliable sensor technology.
Ph is Critical
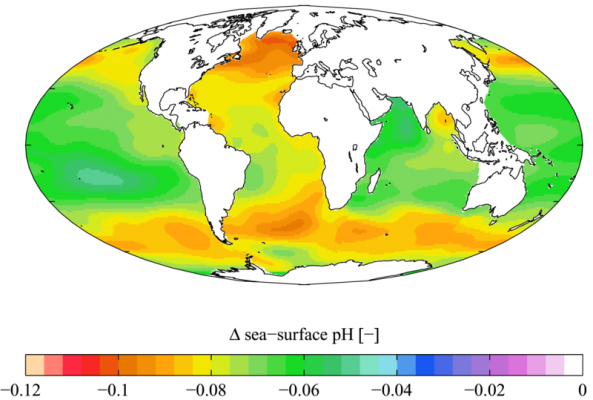
Ocean water is and has been slightly alkaline for a very long time in Earth history. The pre-industrial level of pH in the world’s oceans were 8.179 and during the 1990s it was 8.104, the present level of pH is 8.069, and estimations for 2050 puts it at about 7.949 and in 2100 at 7.824 (see references below), depending on emissions scenario.
The pH value is critical to allow seashells and corals to form. And gradually more acidic oceans have already seen to have real-world implications. As research was done by the National Oceanic and Atmospheric Administration has shown that certain shellfish is having difficulties reproducing when their hatcheries cannot sustain protection enough. Oyster farmers have also repeatedly seen shellfish being decimated by sudden high levels corrosive seawater over the last decade.
This new X-prize competition will result in four finalists, these four will then compete with ocean trials using their developed sensors. Testing accuracy, durability, and flexibility. It should have an ability to network thousands of sensors to monitor conditions on a broad scale.
The prize is also aimed to raise awareness of the problem, as stated by an X-prize spokesman, “We think competitions are a great way to not only deliver us the technology we need but to raise the public’s understanding of this problem,”.
_______________
X-Prize Foundation
______________________________