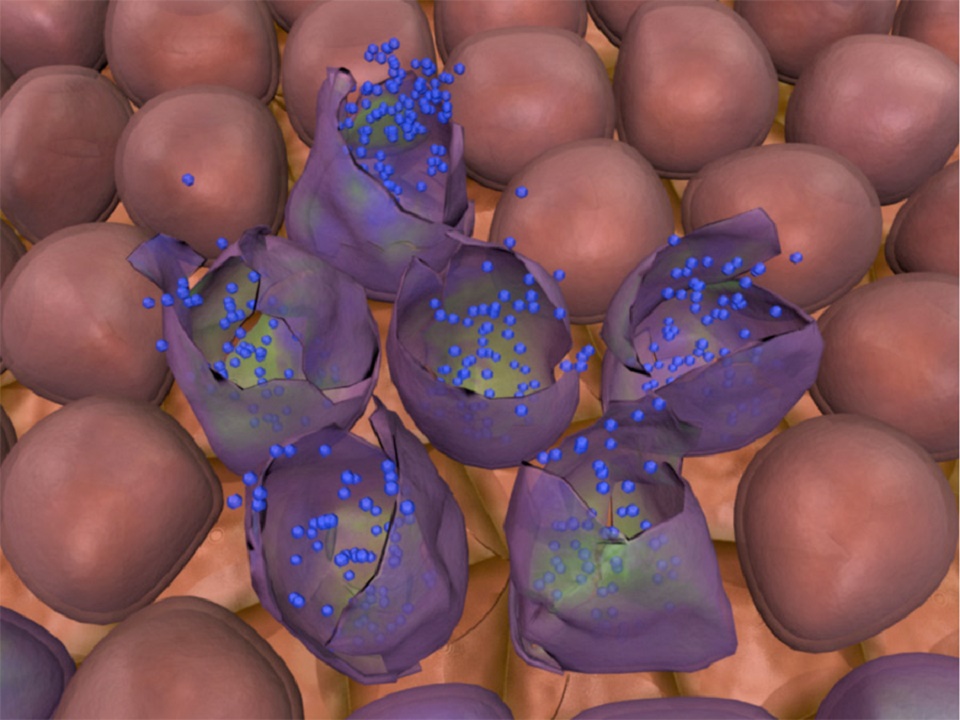
The promising type of future cancer treatment called viral therapy has been envisioned for some time. The idea is to use genetically modified viruses to search and destroy cancer cells.
To intravenously give patients a virus and it would hitchhike the immune system and like a “homing missile” find cancer tumors and destroy them without damaging healthy human tissue.
Much research is underway and earlier this year a study was done at the University of Leeds and The Institute of Cancer Research has shown quite remarkable results.
The study Cell Carriage, Delivery, and Selective Replication of an Oncolytic Virus in Tumor in Patients has been published in Science Magazine.
The research did not intend to measure the effects of treatment on patients’ well-being or disease, but rather to assess the “homing missile” ability of the virus. As the researchers aim to develop a virus that attacks the correct cells in the body without harming other cells.
Ten patients took part in the study, all with advanced bowel cancer and to have it surgically removed. However, metastasis had occurred and tumors had already spread to the liver.
The patients were given up to five doses of a viral therapy during the weeks before surgery. And four weeks after the surgery tests were done to indicate if the virus had any or none effect.
The tissue tests showed that the virus was active in the liver tumor but not in the healthy parts of the liver. This suggests that as intended by design, the virus does target cancer cells after having being injected into the bloodstream.
Dr. Julie Sharp, at Cancer Research UK, said; “This promising study shows that reovirus can trick the body’s defenses to reach and kill cancer cells and suggests that it could be given to patients using a simple injection.”. “We look forward to seeing how this research develops and if this could one day become part of standard cancer treatment.”.
Abstract
Oncolytic viruses are generally designed to be cancer selective on the basis of a single genetic mutation. JX-594 is a thymidine kinase (TK) gene-inactivated oncolytic vaccinia virus expressing granulocyte-macrophage colony-stimulating factor (GM-CSF) and lac-Z transgenes that is designed to destroy cancer cells through replication-dependent cell lysis and stimulation of antitumoral immunity. JX-594 has demonstrated a favorable safety profile and reproducible tumor necrosis in a variety of solid cancer types in clinical trials. However, the mechanism(s) responsible for its cancer-selectivity have not yet been well described. We analyzed the replication of JX-594 in three model systems: primary normal and cancer cells, surgical explants, and murine tumor models. JX-594 replication, transgene expression, and cytopathic effects were highly cancer-selective, and broad spectrum activity was demonstrated. JX-594 cancer-selectivity was multi-mechanistic; replication was activated by epidermal growth factor receptor (EGFR)/Ras pathway signaling, cellular TK levels, and cancer cell resistance to type-I interferons (IFNs). These findings confirm a large therapeutic index for JX-594 that is driven by common genetic abnormalities in human solid tumors. This appears to be the first description of multiple selectivity mechanisms, both inherent and engineered, for an oncolytic virus. These findings have implications for oncolytic viruses in general, and suggest that their cancer targeting is a complex and multifactorial process.
__________________________
Cell Carriage, Delivery, and Selective Replication of an Oncolytic Virus in Tumor in Patients
_____________