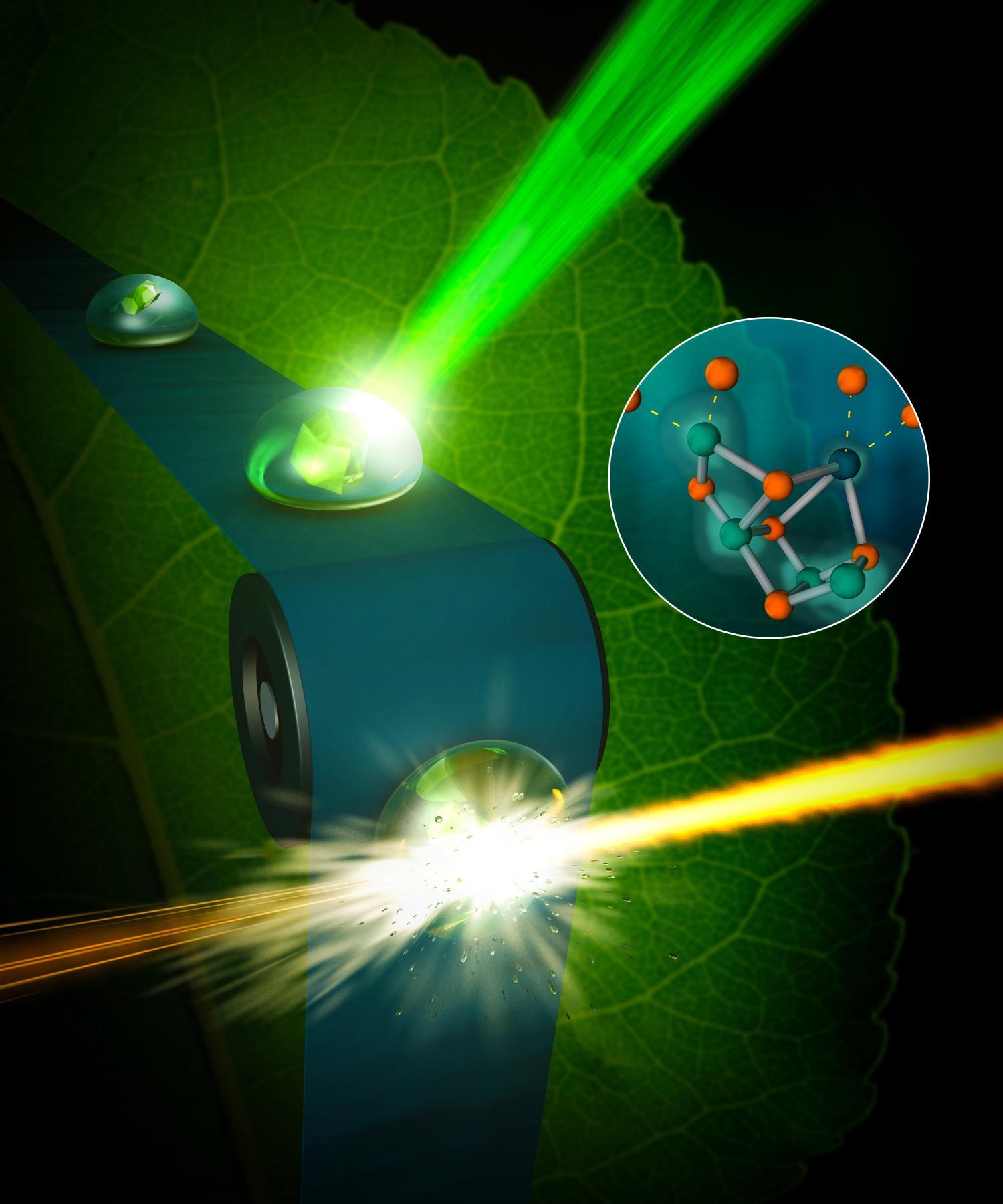
Researchers have long dreamed of replicating the photosynthesis of plants by artificial photosynthesis. A new study might just make this a reality since the researchers have filmed the process.
Photosynthesis, in which solar energy plants convert carbon dioxide and water into sugar and oxygen, is a complicated process, so researchers have not yet managed to copy the process through artificial photosynthesis.
Many believe that artificial photosynthesis may prove very useful in the future since it enables us to harness the energy that flows from the sun could to be used to make fuel and replace today’s fossil fuels without releasing greenhouse gases.
To be able to mimic and replicate photosynthesis, we need to understand in meticulous detail how the process works and a big step in that direction was made just recently when researchers at Berkeley Laboratory in the United States was able to film parts of the photosynthesis process at the micronuclear level.
“The eventual goal is to emulate what photosynthesis has been doing for about three billion years. This has been a research challenge for decades,”
“We now have the right tool, the femtosecond X-ray laser pulses created at LCLS, that allows us to observe the water-splitting reaction as it happens, in real time, and as it happens in nature.”
– Junko Yano, principal investigator and senior scientist at Lawrence Berkeley National Laboratory.
The team filmed the part of the process by which the plant decomposes water using the enzyme “photosystem II, “, in order to gain access to the electrons needed to convert the carbon dioxide into sugar.
Previously, researchers have only been able to capture stills of how it looks after the process is frozen down to extremely low temperatures. But it is now possible to film the process at room temperature, at temperatures when photosynthesis normally work.
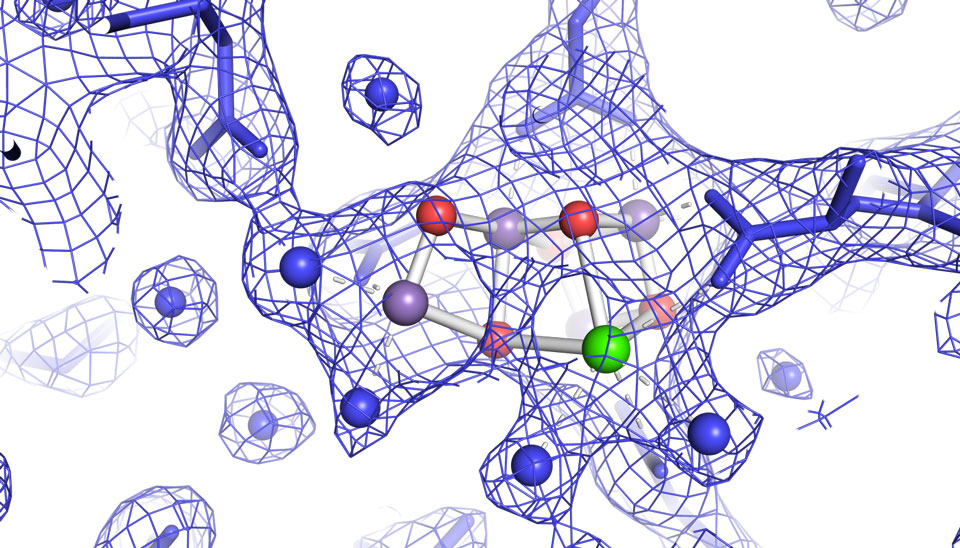
The images, published recently in the journal Nature, provide the first high-resolution 3-D view of photosystem II in action, a feat accomplished by using unimaginably fast X-ray free-electron laser (XFEL) pulses from the Linac Coherent Light Source (LCLS) at SLAC National Accelerator Laboratory, a DOE Office of Science User Facility.
The clips are super short, shorter than one billionth of a second, but the films may nevertheless help scientists to gather more knowledge about how the process works.
Reference:
Iris D. Young, Mohamed Ibrahim et al. Structure of photosystem II and substrate binding at room temperature