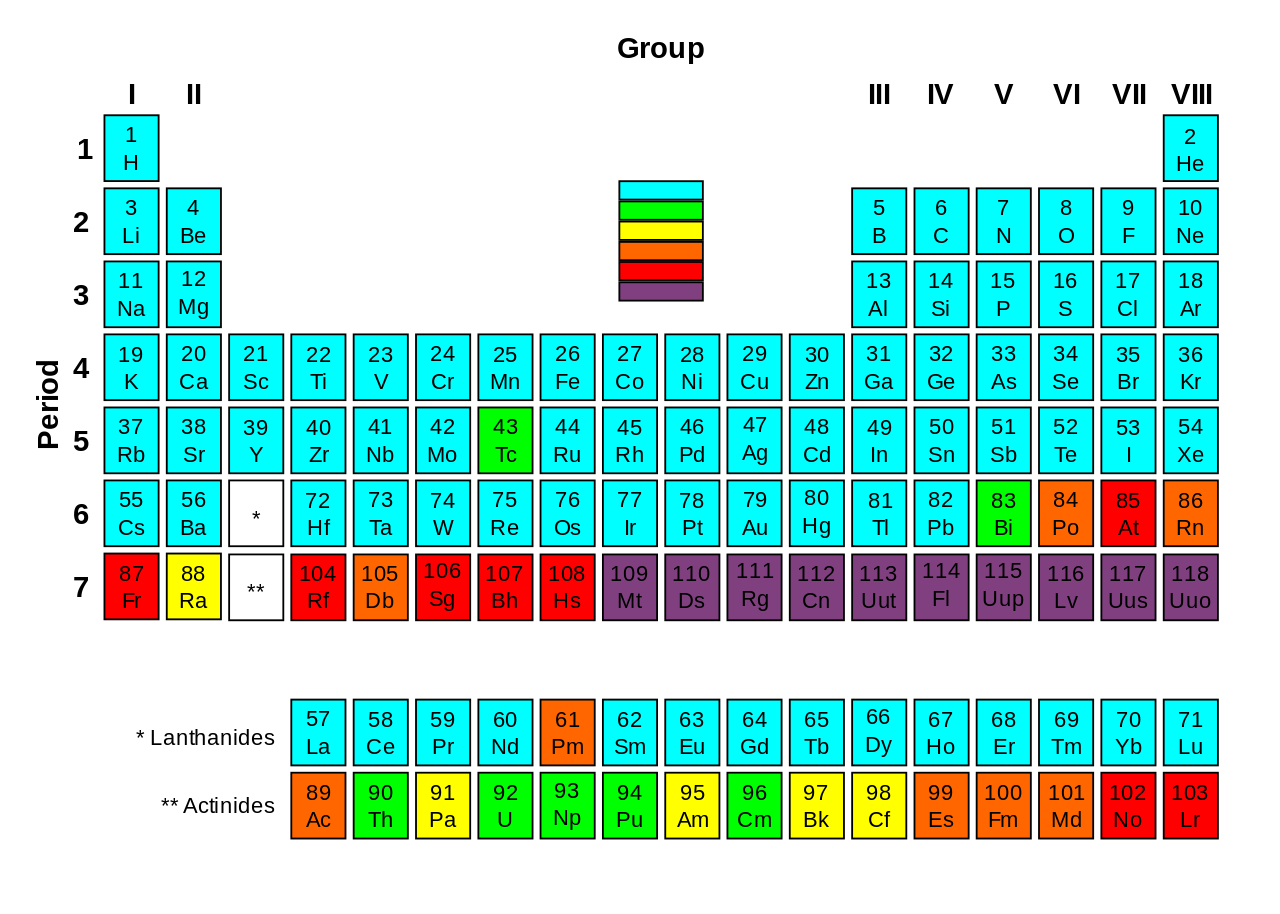
The International Chemistry Union IUPAC have approved four new elements. This approval makes the seventh row of the periodic table complete.
The elements with atomic numbers 113, 115, 117 and 118 are the heaviest known members of the boron group, the nitrogen group, the halogens and noble gasses.
All are highly reactive with half-lives of up to half a second. The elements result from research done by teams in Japan, Russia, and the United States.
The next phase of the process is to name the elements and the scientists behind the discoveries have six months during which they are to suggest names and symbols. These are then to be approved by IUPAC department of inorganic chemistry. Both scientists and the public have a saying on the names before approval by the IUPAC General Assembly in 2017.

They have been given temporary names, though, said Professor Jan Reedijk, President of the Inorganic Chemistry Division of IUPAC. The elements have been temporarily named as ununtrium, (Uut or element 113), ununpentium (Uup, element 115), ununseptium (Uus, element 117), and ununoctium (Uuo, element 118).
Since the periodic table is systematically built, there have been some gaps in the table and these gaps hint of elements that should exist. The elements are not “natural” in the sense of all being created in a laboratory setting. But with this announcement, all the gaps are now filled.
_______________
Discovery and Assignment of Elements with Atomic Numbers 113, 115, 117 and 118
______________________________