Making use of the oceans as an abundant and cheap source of hydrogen is a dream for all those who believe in hydrogen being the future fuel of choice and a general source of energy.
Recently a study was published in Nature Chemistry presenting a new catalyst that could imply a technological leap for hydrogen production from water. The new catalyst is so cheap it can compete with hydrogen produced from fossil fuels.
Water is usually separated into hydrogen and oxygen through a process called electrolysis, applying electrical currents via a series of immersed electrodes, these electrodes act as chemical catalysts for the reaction (2 H2O(l) → 2 H2(g) + O2(g)).
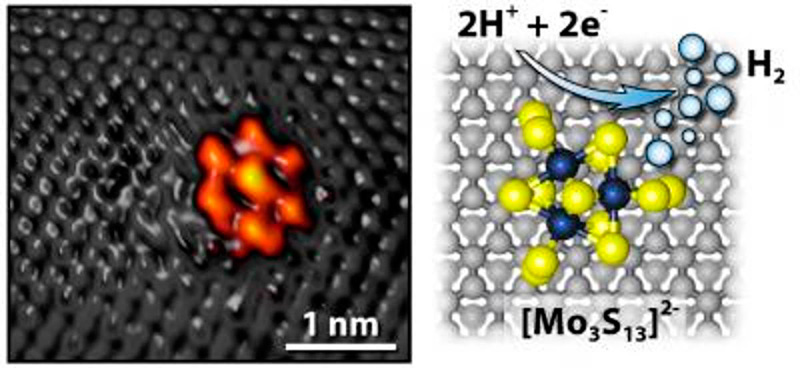
This new catalyst is a novel approach made using a nanocluster of molybdenum sulfide (MoS2) attached to a surface of graphite. The electrolysis catalyzed by the use of molybdenum sulfide attached to graphite makes for the reaction when two hydrogen ions each receive an electron when they come in contact with the molybdenum sulfide (see featured image), and then forms a hydrogen molecule. It is a new method of breaking the bonds between hydrogen (H2) and oxygen (O) in water.
Molybdenum sulfide has not been known for this catalytic property previously. It has been used to refine oil since the Second World War. But the scientists now managed to create several new properties by changing the molecular structure of the molybdenum sulfide and then attach it to the graphite.
Platinum is generally regarded as the ideal material for such electrodes, but with its price being very high, a device made with platinum cannot compete with other methods to produce hydrogen, such as producing hydrogen from methane. This is a dirty process though and carbon dioxide is created in the process.
In order to compete with hydrogen produced from fossil fuels, the costs must come down to between 1 and 2 dollars per kilo of hydrogen and the current production costs using this new method would be between 1,6 and 10,4 dollars per kilo, according to the researchers.
If hydrogen can be produced in a cheap and efficient way from water, it would potentially imply a totally clean cycle of water. Hydrogen fuel cells make use of hydrogen to produce energy, with water being the only waste product.
The research has been done by teams at Stanford University in the United States and the University of Aarhus in Denmark. Thomas Jaramillo is a professor of chemical engineering at Stanford University, “There are many pieces of the puzzle still needed to make this work, and much effort ahead to realize them. However, we can get huge returns by moving from carbon-intensive resources to renewable, sustainable technologies to produce the chemicals we need for food and energy,”.
_______________
Nature: Building an appropriate active-site motif into a hydrogen-evolution catalyst with thiomolybdate [Mo3S13]2− clusters
Stanford University Press Release
______________________________